Navigator Workbench
Streamline your drug development process
The benchmark in PK/PD modelling
The drug development process is costly and time consuming. But it is essential. Navigator Workbench streamlines PK/PD modelling to help pharmaceutical and clinical research organisations make better decisions faster – while reducing the risk of human error.
MODELLING THE FUTURE
How can we bring drugs to market cost-effectively? How can we use approved drugs to make therapy more effective? What’s the fastest and most reliable way to model new treatments?
Navigator Workbench (including ModSpace – our analytic and model management platform) provides a validated environment for PK/PD model storage, development, execution, analysis and reporting. It supports end-to-end modelling activities including automatically generating FDA 21 CFR Part 11 compliant reports. Developed with clinical modelling teams, Navigator Workbench provides a complete Modelling and Simulation solution.
A PORTAL TO COLLABORATION
Navigator Workbench provides a centralised and collaborative portal for NONMEM based projects, allowing distributed modelling teams to store, interrogate, visualise and report on model development activities.
Users get controlled access to a high-performance computing grid, a suite of customisable reports, and model evaluation tools. Admins can manage permissions and roles as part of the QC process, while integrated workflows promote standardisation and best practice.
That’s why global pharmaceutical and clinical research organisations depend on Navigator Workbench to obtain valuable insights into new drug development, while enhancing collaboration, reducing risk and improving productivity.
Key benefits
- Connect teams – stimulate collaboration across distributed teams via a single central storage point. A powerful built-in search engine locates desired artefacts quickly and easily.
- Reduce risk – during PK/PD model development by using a single platform.
- Standardise workflow and promote best practice – tools and environment to support end-to-end modelling.
- Achieve regulatory compliance – out-of-the-box support for over 100 standard reports, which can be included in FDA 21 CFR Part 11 compliant final submissions.
- Ensure access to all – user-friendly GUI and pre-configured job execution profiles support novice users whilst power users can leverage more complex commands.
Key features
- Develop – Create and edit NONMEM Models and scripts in a number of supported languages, using built-in file editors.
- Execute -Execute NONMEM, R, SAS, MATLAB and PsN jobs and scripts on a High-Performance Computing Grid.
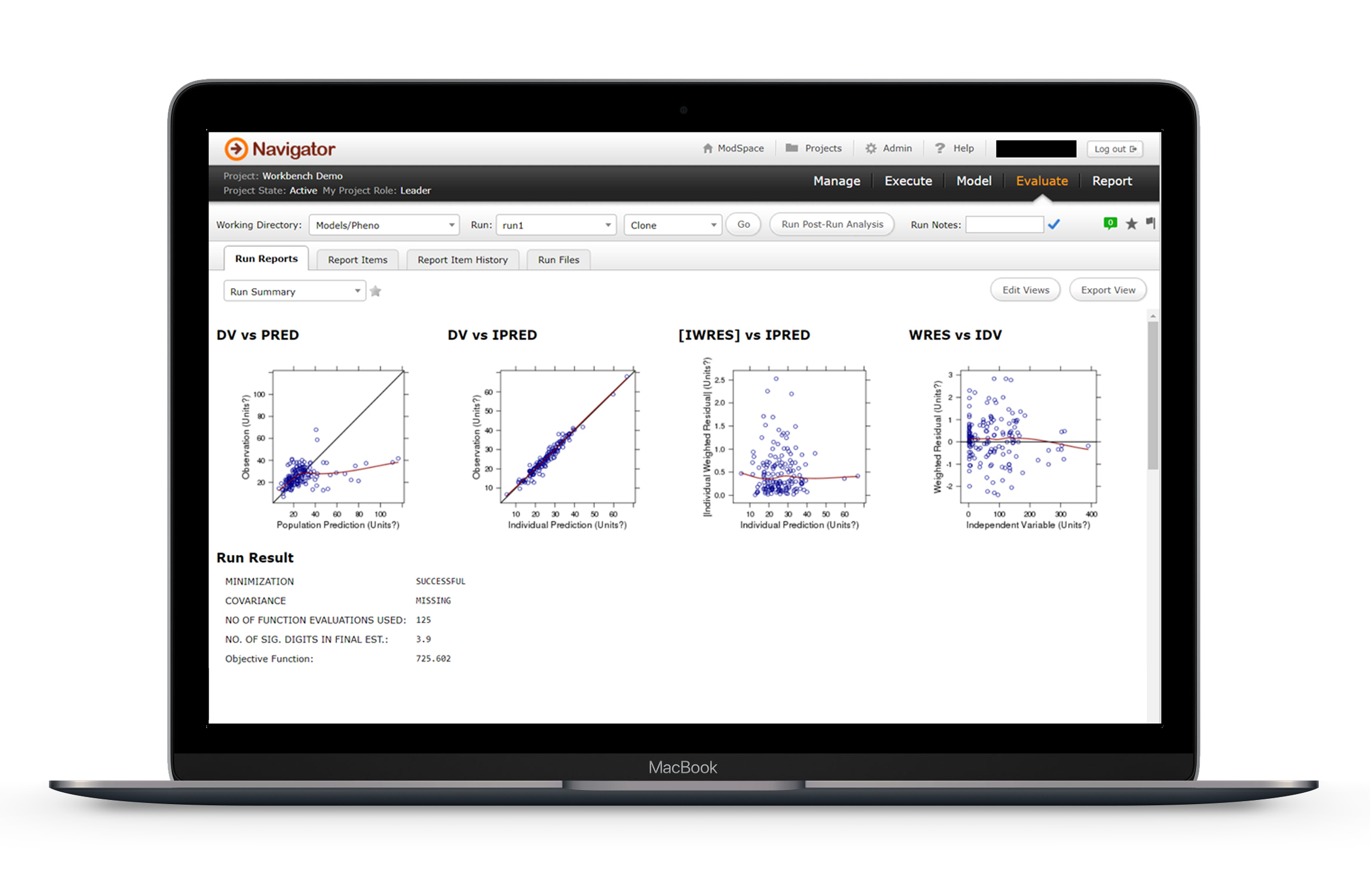
- Analyse – Explore and compare results and model evolution with Tree or Tabular viewing options. Perform further analysis of NONMEM Models using “Post Run Analysis” offered by PsN, such as Bootstrap, VPC and SCM. Produce adjustable diagnostic plots and tables to assess model fit.
- Share and Collaborate – Share modelling activities and results while managing project permissions and team roles as part of a QC process. Security and controlled access allow for blinded and due diligence modelling projects.
- Report – Generate publication-ready standard and bespoke reports and publish to various document output formats.
- Store and Audit – Confidently store all project content in our version-controlled repository, fully assured that all required modelling activities are audited and fully compliant.
Resource
Explore the latest insights to inspire your next data science project.
Contact Us
Contact us for more information or to arrange a demo of Navigator Workbench.
Get in touch